Introduction: Sickle cell disease (SCD) is an inherited, multisystem blood disorder that affects millions of people worldwide and is particularly common among those of African descent. Vaso-occlusive crises (VOCs) or acute pain events involving complex interactions among sickled erythrocytes, endothelial cells, leukocytes, and activated platelets are hallmark symptoms of SCD. Over time, episodic bouts of acute VOC may develop into chronic pain, defined as pain most days for 6 months. At present, there are no biomarkers that distinguish between acute and chronic pain. Here, we explore differences in gene expression during VOC events in a cohort of pediatric patients with SCD. The objective of this study was to examine transcriptome alterations in CD45+ cells of individuals with SCD during a VOC event compared with steady state (SS) in patients with or without chronic pain.
Methods: Patients were aged <21 years, with HbSS or HbSβ 0 genotypes. Informed consent was obtained for all patients. CD45+ cell (lymphohematopoietic lineage) samples were collected from 158 patients with SCD under SS conditions (at the maximum tolerated dose of hydroxyurea; some patients were also on metformin) during a VOC event, and at a follow-up visit after a VOC. RNA sequencing (RNA-Seq) was performed using a 101-base pair, paired-end protocol run on the NovaSeq 6000 system. We applied supervised normalization of microarrays to adjust the normalized gene expression values for the effect of multiple covariates. ToppGene Suite was used for over-representation analysis (ORA; Chen et al. Nucleic Acid Res 2009). Analysis of differentially expressed genes was performed using limma software and gene set enrichment analysis using the Molecular Signature Database.
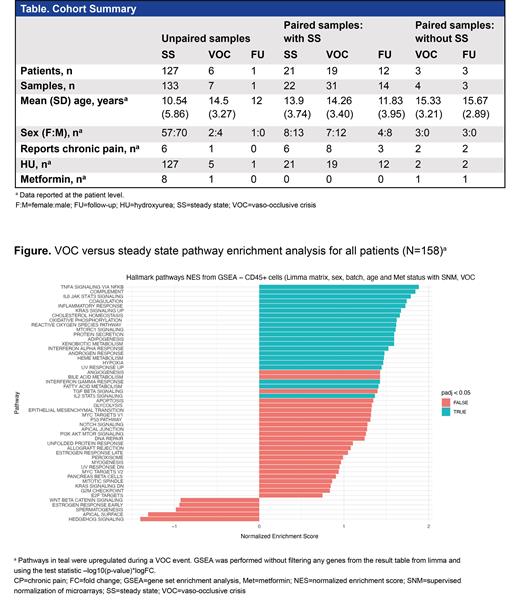
Results: A summary of the study cohort is presented (Table). Most patients (98.7%) were on hydroxyurea. We collected RNA-Seq data from unpaired and paired samples at SS and during a VOC. After adjusting for age, sex, chronic pain status, and metformin status, analyses of RNA-Seq data showed upregulated genes linked to TNFα signaling via NFΚB, IL-6/JAK/STAT3 signaling, complement activation, and coagulation were enriched in CD45+ cells during VOCs compared with SS (Figure). Differentially expressed genes were also compared with whole blood transcriptome samples collected at baseline and during a VOC event as reported by Creary et al (Sci Rep 2020). Approximately 290 upregulated and 33 downregulated genes were shared in both studies. ORA analysis of the shared genes revealed upregulation of pathways such as neutrophil degranulation and complement activation during a VOC event. Analysis of samples from patients without chronic pain at SS indicated similar immune-related pathways, such as complement activation and interferon α response, were upregulated during a VOC event. In contrast, analysis of samples from patients reporting chronic pain revealed no significant differences in gene expression between a VOC event and SS.
Conclusions: Samples collected during a VOC event showed significant changes in the transcriptome of CD45+ cells compared with SS in pediatric patients who had the most severe disease, or sickle cell anemia characterized by HbSS and HBSβ 0/+ genotypes, without a diagnosis of chronic pain, with multiple pathways altered by VOC. However, when VOC transcriptomes in patients with chronic pain were compared with SS, these pathways were not significantly altered. Our pediatric patient population had low rates of chronic pain; therefore, our sample size was limited. Additional analyses of SS and VOC transcriptomes, particularly in patients with chronic pain, will expand our understanding of the impact of VOC events on transcriptomes in individuals with and without chronic pain. This may lead to identification of quantitative biomarkers for a VOC, facilitating testing of new therapies for the treatment of pain in people with SCD.
Disclosures
Potdar:Pfizer: Current Employment. Yu:Pfizer: Current Employment. Sheehan:Refoxy Pharmaceuticals: Research Funding; NHLBI TOPMed program: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Beam Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal